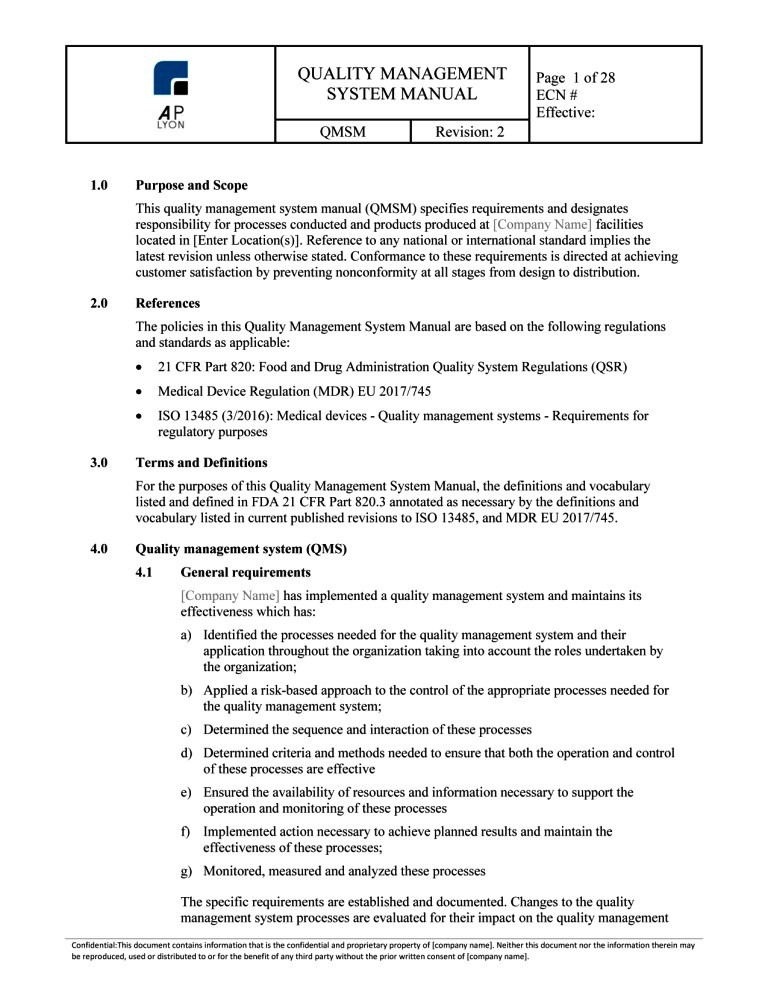
Iso 13485 Quality Manual Template Free
Iso 13485 quality manual template free - Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA. GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline. He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. It was just good practice. Despite being named ISO 9000 this template is in fact built with the ISO 9000 family for quality management systems in mind and as such can be used for ISO 9001. Fortunately IMRDF or GHTF created a template called STED Summary Technical Documentation medical device to help organize all the information but this was not mandatory per legislation. Our core and safety processes as defined in the quality management manual must be reviewed at minimum once per year. Discussion Threads 307 Posts 4367. GMP Certificate can be issued by Government organization and Certification Bodies. This is a free template provided by OpenRegulatory.
The purpose of this mini. Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001. SOP Document and Record Control. Automotive News TISAX - VDA ISA. First you need to know that the EU MDR 2017745 is providing a clear view of what should contain a technical file when the MDD 9342EC was not so structured.
Does Your Iso 13485 Quality Manual Looks Like That Pdf Example
GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA.
Show ImageSample Iso 13485 Quality Manual Procedures Package
This is a free template provided by OpenRegulatory. ISO 134852016 Section Document Section. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP.
Show ImageHow To Write A Quality System Plan Template Free Download Medical Device Academy
All other processes. GMP Certificate can be issued by Government organization and Certification Bodies. Our core and safety processes as defined in the quality management manual must be reviewed at minimum once per year.
Show ImageIso 13485 2016 Writing A Short Quality Manual
QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. Quality Manual Development and Quality Systems Manual related Discussions. Automotive News TISAX - VDA ISA.
Show ImageIso 13485 Quality Management System Manual
GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline. This ISO 9001 structure template offers a concise and easy-to-follow framework for creating a quality management system QMS mini-manual that complies with ISO 9001 requirements. SOP Document and Record Control.
Show ImageIso 13485 Quality Manual Sample Pdf Quality Management System Iso 9000
Quality Manual Development and Quality Systems Manual related Discussions. Discussion Threads 307 Posts 4367. Free CIOB - Code of Quality Management.
Show ImageIso 13485 2016 Quality Manual And Procedures Iso 13485 Store
GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline. This is a free template provided by OpenRegulatory. Quality assurance document control is the process used in the management coordination control delivery or support of an item required for quality assurance purposes.
Show ImageIso 13485 Quality Manual For Medical Devices
Automotive News TISAX - VDA ISA. 424 All 425 All. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA.
Show ImageImsxpress Iso 13485 Template Documentation Qms Management Software
He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. Free CIOB - Code of Quality Management. It was just good practice.
Show ImageSample Iso 13485 Quality Manual Procedures Package
GMP Certificate can be issued by Government organization and Certification Bodies. ISO 134852016 - Medical Device Quality Management Systems. He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review.
Show ImageDiscussion Threads 307 Posts 4367. Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. ISO 134852016 Section Document Section. GMP Certificate can be issued by Government organization and Certification Bodies. This ISO 9001 structure template offers a concise and easy-to-follow framework for creating a quality management system QMS mini-manual that complies with ISO 9001 requirements. ISO 134852016 - Medical Device Quality Management Systems. 424 All 425 All. Free CIOB - Code of Quality Management. He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review.
Quality assurance document control is the process used in the management coordination control delivery or support of an item required for quality assurance purposes. This is a free template provided by OpenRegulatory. Despite being named ISO 9000 this template is in fact built with the ISO 9000 family for quality management systems in mind and as such can be used for ISO 9001. Automotive News TISAX - VDA ISA. Our core and safety processes as defined in the quality management manual must be reviewed at minimum once per year. Fortunately IMRDF or GHTF created a template called STED Summary Technical Documentation medical device to help organize all the information but this was not mandatory per legislation. First you need to know that the EU MDR 2017745 is providing a clear view of what should contain a technical file when the MDD 9342EC was not so structured. The purpose of this mini. All other processes. GMP Certificate helps your organization to ensure regulatory compliance while demonstrating your knowledge and commitment to produce safe quality healthcare products to the public.
SOP Document and Record Control. GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline. Quality Manual Development and Quality Systems Manual related Discussions. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA. It was just good practice.